Abstract
Background: Myelofibrosis (MF) is a disorder characterized by unrestrained proliferation of myeloid precursors and dysfunctional Janus kinase (JAK) signaling. Hyperplastic and dysplastic megakaryocyte expansion in MF releases inflammatory cytokines. These cytokines promote fibroblast proliferation, bone remodeling, and marrow fibrosis, disrupting the osteohematopoietic niche and leading to ineffective and extramedullary hematopoiesis (Vainchenker et al. 2017). Clinical manifestations include splenomegaly, anemia, and often disabling constitutional symptoms. JAK inhibitors, such as ruxolitinib, have been shown to improve spleen size and symptoms, but are associated with dose-limiting anemia and thrombocytopenia, reducing their utility for many patients (Verstovsek et al. 2012).
KER-050 is an investigational product designed to alter signaling of TGF-β superfamily ligands, restoring balance to the osteohematopoietic niche and promoting differentiation of erythroid and megakaryocytic precursors. It is a modified activin receptor type IIA ligand trap that inhibits TGF-β superfamily ligands, including activins A and B and growth and differentiation factors (GDFs) 8 and 11. Preclinical studies have demonstrated that KER-050 induces erythropoiesis, increases platelet counts, improves bone mass, and prevents fibrosis. A preclinical study previously presented demonstrated that RKER-050 can reverse ruxolitinib-associated reductions in hemoglobin, hematocrit, and red blood cell count, indicating potential for KER-050 to mitigate ruxolitinib toxicity. In two clinical studies, including an ongoing study in participants with myelodysplastic syndromes (MDS), KER-050 has been well-tolerated. Accumulated clinical data demonstrate that KER-050 can stimulate erythropoiesis and thrombopoiesis in both healthy post-menopausal women and MDS participants with ineffective hematopoiesis. Here we present data from the first clinical study of KER-050 in participants with MF (NCT05037760). The objective of this study is to evaluate the potential of KER-050 as monotherapy and in combination with ruxolitinib to treat the underlying pathology of MF and mitigate JAK inhibitor-associated cytopenias.
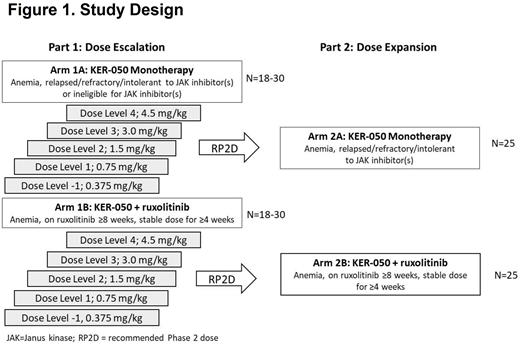
Methods: This ongoing Phase 2 open-label study is evaluating the safety, tolerability, pharmacokinetics PK), pharmacodynamics (PD), and efficacy of KER-050 administered with or without ruxolitinib in participants with primary myelofibrosis (PMF), post-essential thrombocythemia myelofibrosis (post-ET MF), and post-polycythemia vera myelofibrosis (post-PV MF) who have anemia (hemoglobin ≤ 10 g/dL or receiving transfusions). Part 1 involves parallel dose escalation arms (1A: monotherapy, 1B: combination with ruxolitinib) to evaluate the safety and tolerability of KER-050 and identify the dose(s) of KER-050 to be evaluated in Part 2 (Figure 1). Changes in hemoglobin, transfusion burden, spleen size, the Myelofibrosis Symptom Assessment Form Total Symptom Score (MF-SAF-TSS), PK and incidence of accelerated MF and acute myeloid leukemia are assessed as secondary endpoints. This study is also evaluating markers of multilineage hematopoiesis, bone remodeling, iron metabolism, inflammation, and marrow fibrosis. Frequency of ruxolitinib dose modification and quality of life will be explored.
Results: Preliminary results from Part 1 will be presented. At the time of this writing, 5 patients have received KER-050 on study, 3 in Arm 1A and 2 in Arm 1B. Three participants have completed the dose-limiting toxicity (DLT) assessment period and no DLTs have been experienced. Data evaluated to date indicate a PD effect consistent with previous preclinical and clinical studies of KER-050.
Summary: KER-050 is designed to alter TGF-β superfamily signaling, restoring balance to the osteohematopoietic niche and promoting multilineage hematopoietic differentiation. Here we present data from the first study of KER-050 in participants with MF evaluating the potential for KER-050 to impact key aspects of the MF disease state (anemia, spleen size, symptoms, progression), as monotherapy and in combination with ruxolitinib. The potential for KER-050 to mitigate ruxolitinib toxicity and related dose-reductions is being investigated. Exploratory analyses of the KER-050 impact on inflammation, bone remodeling, iron metabolism, and marrow fibrosis, as available, will be shared.
Disclosures
Harrison:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Sierra: Honoraria; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Promedior: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; EHA: Other: Leadership role; MPN voice: Other: Leadership role; Galacteo: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding. Ross:Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Celgene: Research Funding; BMS: Honoraria. McGinty:Vertex Pharmaceuticals: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Keros Therapeutics: Current Employment; Biogen: Current equity holder in publicly-traded company. Feng:Keros Therapeutics: Current Employment; Biogen: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Jiang:Repare Therapeutics: Ended employment in the past 24 months; Alkermes, Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Keros Therapeutics: Current Employment. Graham:Alkermes, Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Keros Therapeutics: Current Employment. Rovaldi:Keros Therapeutics: Current Employment. Cooper:Anokion Therapeutics: Current equity holder in private company, Ended employment in the past 24 months; Keros Therapeutics: Current Employment; Kadmon Corporation: Ended employment in the past 24 months. Salstrom:Keros Therapeutics: Current Employment; Homology Medicines, Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal